Introduction to Freeze Drying Specialty Chemicals
The preservation and stabilization of high-value, sensitive materials are paramount across numerous advanced industries. Freeze drying, also known as lyophilization, stands out as a critical technique for achieving this, particularly in the realm of specialty chemicals.
Brief Overview of Freeze Drying (Lyophilization) and Its Principles
Freeze drying is a gentle dehydration process used to preserve perishable materials or to make the material more convenient for transport and storage. The fundamental principle relies on sublimation, where ice converts directly into water vapor under a vacuum without passing through a liquid phase.
The process consists of three main stages:
- Freezing: The product is cooled below its eutectic or glass transition temperature to completely solidify all its components.
- Primary Drying (Sublimation): Under a deep vacuum, controlled heat is applied to allow the frozen solvent (typically water) to sublime.
- Secondary Drying (Desorption): The temperature is raised further, and the vacuum maintained to remove residual, adsorbed moisture, resulting in a product with a very low moisture content.
Importance and Benefits of Freeze Drying in the Specialty Chemicals Industry
For sensitive specialty chemicals—which often include complex organic molecules, biological compounds, and advanced materials—conventional drying methods involving high heat or simple evaporation can lead to degradation, loss of activity, or undesirable physical changes.
Freeze drying is essential because it offers a highly controlled, low-temperature environment that preserves the chemical structure, biological activity, and morphology of the initial material.
Key benefits of applying freeze drying to specialty chemicals include:
- Improved Stability and Increased Shelf Life: By reducing the moisture content to <1-3%, critical degradation pathways—especially those catalyzed by water—are significantly slowed, drastically extending the product's usable life.
- Enhanced Solubility (Reconstitution): The resulting porous, high-surface-area lyophilized cake often dissolves quickly and completely upon the addition of a solvent, which is crucial for injectables and diagnostic reagents.
- Precise Control over Particle Size and Morphology: The freezing and drying protocols can be finely tuned to influence the final product’s physical characteristics, which is vital for performance in advanced materials and nanomaterials.
- Simplified Storage and Transport: The reduced weight and volume of the dried product, coupled with its enhanced stability at ambient or refrigerated temperatures, lower logistics costs and risks.
Understanding Specialty Chemicals
To effectively utilize freeze drying equipment, one must first understand the nature of specialty chemicals and the unique demands they place on the process.
Definition and Characteristics of Specialty Chemicals
Specialty chemicals, also known as performance chemicals, are specific chemical products sold on the basis of their performance or function, rather than their composition alone. They are generally high-value, low-volume products with complex formulations and specific purity requirements.
Key Characteristics:
- Functionality-Driven: Their value is derived from their specific effect (e.g., catalyzing a reaction, providing a therapeutic effect, or enabling detection).
- High Sensitivity: Many specialty chemicals, especially biologicals like enzymes and protein therapeutics, are highly sensitive to heat, shear stress, and water activity, which can lead to denaturation or degradation.
- Purity Requirements: Applications in regulated industries like pharmaceuticals and diagnostics demand extremely high levels of purity, often necessitating aseptic processing.
- Complex Formulations: They frequently require the inclusion of excipients, buffers, and cryoprotectants (sucrose, trehalose, mannitol) to maintain stability during freezing and drying.
Examples of Specialty Chemicals Suitable for Freeze Drying
The gentle, low-temperature nature of lyophilization makes it ideal for a wide range of sensitive, high-value materials:
| Specialty Chemical Category |
Examples and Entities |
Why Freeze Drying is Used |
| Pharmaceuticals |
Vaccines, injectables, protein therapeutics, antibodies |
Preserves biological activity, extends shelf life, and allows for easy reconstitution in clinical settings. |
| Diagnostics |
Enzymes, antibodies, calibrators |
Maintains the precise activity and structural integrity required for accurate analytical performance. |
| Probiotics |
Live bacterial cultures (microorganisms) |
Preserves cell viability and stability without using damaging heat, ensuring a potent final product. |
| Enzymes |
Industrial and biological catalysts |
Maintains tertiary structure and catalytic activity, which would be destroyed by conventional heat drying. |
| Nanomaterials |
Liposomes, nanoparticles, advanced drug delivery vehicles |
Prevents particle agglomeration during drying, creating stable dispersions and controlled particle size. |
Unique Challenges and Considerations When Freeze Drying Specialty Chemicals
The complexity and sensitivity of these materials introduce significant challenges that necessitate precise control over the lyophilization cycle:
- Low Collapse/Eutectic Temperatures: Many formulations have very low critical temperatures, meaning the product must be kept extremely cold during primary drying to prevent the structure from collapsing.
- Product Heterogeneity: Complex mixtures (e.g., diagnostic calibrators or multi-component vaccines) require process parameters that accommodate the different critical temperatures of various components.
- Residual Moisture Tolerance: For long-term stability, particularly of biologics, the final moisture content must be extremely low (often <1%), requiring extended secondary drying.
- Scale-Up Complexity: Transitioning a successful lab-scale formulation to a production freeze dryer is challenging, as mass and heat transfer dynamics change significantly. This requires robust engineering expertise and data-driven modeling.
Addressing these challenges is where specialized knowledge and advanced equipment become non-negotiable. Sieno Freeze-drying Technology Research Institute (Jiangsu) Co., Ltd, for example, focuses on the profound integration of freeze-dried food science with intelligent equipment manufacturing. While known for driving innovation in food processing, Sieno's commitment to consolidating world-foremost freeze-drying technology resources, engaging in strategic collaborations with university experts, and leveraging independently developed intelligent freeze-drying equipment provides the foundational technological expertise critical for handling the precise demands of sensitive specialty chemicals. This synergy ensures that the equipment can handle complex thermal profiles and maintain the strict environmental controls required for high-value applications.
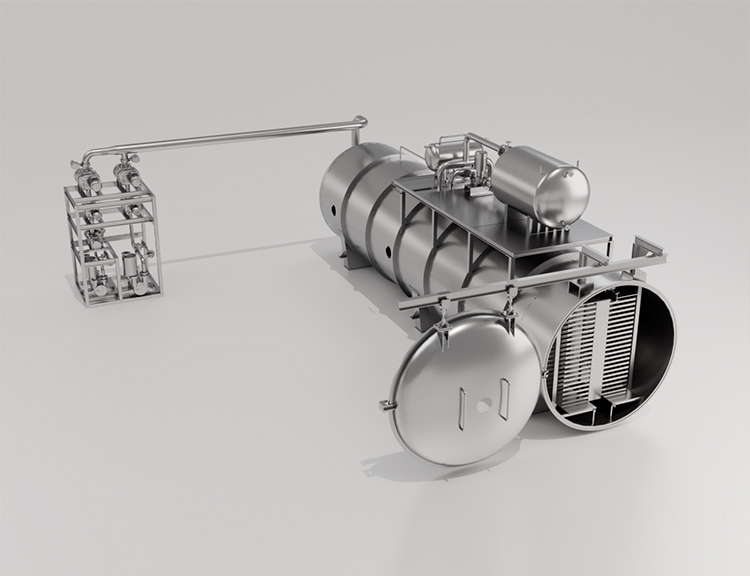
Freeze Drying Equipment for Specialty Chemicals
The success of freeze drying specialty chemicals hinges on selecting and operating the right freeze drying equipment. This machinery must provide precise, reproducible control over temperature and vacuum to protect sensitive formulations.
Types of Freeze Dryers
Freeze dryers are categorized primarily by their capacity and intended application, reflecting the different stages of product lifecycle from discovery to commercialization:
| Type of Freeze Dryer |
Typical Application |
Key Characteristics and Scale |
| Laboratory Freeze Dryers |
R&D, feasibility studies, formulation screening, small-batch testing. |
Benchtop or small-scale, easy portability, designed for rapid testing, capacity typically <10 L of ice. |
| Pilot-Scale Freeze Dryers |
Scale-up studies, process optimization, clinical trial material production. |
Intermediate size, high level of instrumentation and control, capacity typically 10-50 L of ice. Essential for collecting data needed for production models. |
| Production Freeze Dryers |
Large-scale commercial production, cGMP environments. |
Maximum capacity (up to hundreds of liters of ice), full automation, designed for continuous high throughput, fully integrated Clean-in-Place (CIP)/Sterilize-in-Place (SIP) features. |
Key Components of a Freeze Dryer
Regardless of scale, all professional freeze dryers share core systems engineered to achieve and maintain the extreme conditions necessary for sublimation:
- Vacuum System: Essential for lowering the chamber pressure far below the triple point of water.
- Function: Creates the deep vacuum required to facilitate the sublimation of ice directly into vapor.
- Performance Metric: Vacuum performance and leak tightness are critical to prevent air infiltration and maintain low pressure during the entire drying cycle.
- Refrigeration System: Responsible for cooling the product and the ice condenser.
- Function: Cools the product below its freezing point (often to −40∘C or lower) and cools the condenser (often to −70∘C to −85∘C) to trap the water vapor.
- Heating System (Shelf System): Provides the necessary latent heat for the sublimation process.
- Function: Circulates a heat transfer fluid (e.g., silicone oil) through the shelving system to deliver controlled energy to the frozen product.
- Critical Feature: Temperature control and uniformity across all shelves are vital for consistent product quality and preventing hot spots that could cause product collapse.
- Control System: The 'brain' of the operation.
- Function: Monitors and adjusts process parameters (shelf temperature, chamber pressure, condenser temperature) in real-time. Crucial for process optimization and ensuring the product stays within its critical temperature limits. Includes data logging features for regulatory compliance.
Factors to Consider When Selecting Specialty Chemicals Freeze Drying Equipment
Selecting the correct equipment is a decision that affects process efficiency, product quality, and regulatory compliance.
| Factor |
Critical Consideration for Specialty Chemicals |
| Capacity and Throughput |
Must align with expected batch size and annual production demand. Over/undersizing impacts capital and operating costs. |
| Temperature Control and Uniformity |
Required temperature range must exceed the critical temperatures of the formulation. Uniformity is essential for batch consistency. |
| Vacuum Performance & Leak Tightness |
The ability to achieve and hold a low absolute pressure (e.g., $10$ to $100$ mTorr) for effective sublimation of complex solvents. |
| Sterilization and Cleaning Capabilities |
For pharmaceuticals and diagnostics, CIP/SIP capabilities are often mandatory to prevent cross-contamination and meet cGMP standards. |
| Automation and Data Logging |
Advanced control systems (SCADA/PLC) are needed for reproducible cycles and detailed quality control records. |
| Regulatory Compliance (cGMP) |
Equipment must be designed, manufactured, and documented to meet strict regulatory requirements (e.g., FDA, EMA) for material traceability and operation. |
Sieno Freeze-drying Technology Research Institute (Jiangsu) Co., Ltd, leveraging its focus on the profound integration of science with intelligent equipment manufacturing, offers tailored solutions that directly address these selection factors. By utilizing their independently developed intelligent freeze-drying equipment and collaborating with experts to consolidate world-foremost freeze-drying technology resources, Sieno ensures their systems deliver the precise temperature control, robust vacuum integrity, and advanced automation necessary to stabilize the most sensitive specialty chemicals while meeting stringent industry demands for scale-up and cGMP compliance.
The Freeze Drying Process for Specialty Chemicals
The freeze drying process for specialty chemicals is a multi-step, tightly controlled sequence designed to remove water while preserving the activity and structure of sensitive materials. Precision in each stage is vital for achieving a high-quality final product.
Pre-Freeze Drying Steps
Before the product enters the freeze drying equipment, careful preparation is essential to ensure the success of the entire cycle.
- Formulation: This is the most critical step. It involves selecting the primary solvent (usually water) and incorporating necessary additives.
- Cryoprotectants and Stabilizers: Additives like sucrose, trehalose, or mannitol are chosen to protect the material (e.g., protein therapeutics or enzymes) during the freezing and drying stress, preventing denaturation and maintaining stability.
- Solute Concentration and pH: These factors significantly influence the product's critical temperatures (eutectic or glass transition) and, thus, the subsequent drying parameters.
- Filtration: Solutions must be filtered to remove particulate matter and, particularly for injectables and vaccines, to ensure sterility. This is often done using sterile-grade membrane filters (0.22μm).
- Filling: The liquid product is dispensed into the final container, such as vials, trays, or ampoules. Uniformity of fill volume is crucial, as it affects the heat transfer dynamics during drying.
Freezing Stage
This stage sets the physical structure of the product by converting the liquid solvent into a solid ice matrix.
- Controlled Cooling Rates: Fast or slow cooling rates impact the size of the resulting ice crystals.
- Slow Cooling: Tends to produce fewer, larger ice crystals, which can create larger pores in the final cake, potentially leading to faster subsequent drying but also possible phase separation.
- Fast Cooling (Quenching): Tends to produce many small, uniform crystals, which is often preferred for sensitive nanomaterials to maintain particle integrity, but can result in longer drying times.
- Annealing (Optional): This involves cycling the temperature above the critical freezing point for a short duration and then re-cooling. Annealing can improve the ice crystal structure, promote homogeneity, and increase the critical temperature of the frozen matrix, facilitating better sublimation.
Primary Drying Stage (Sublimation)
This is the longest stage, where the bulk of the water (as ice) is removed. The product must remain below its critical collapse temperature.
| Parameter |
Goal and Impact |
Comparison/Range |
| Chamber Pressure (Vacuum) |
Controlled to allow sublimation. Lower pressure increases the driving force. |
Typically 50 to 200 mTorr (or 6.7 to 26.7 Pa) |
| Shelf Temperature (Heat Input) |
Provides the latent heat for sublimation. Must be carefully controlled to keep the ice interface temperature below the collapse temperature. |
Varies widely, often −25∘C to 0∘C during this phase. |
| Product Temperature |
The most critical metric. Must be continuously monitored using thermocouples to ensure it never exceeds the material's critical temperature (e.g., glass transition). |
Should be 2∘C to 5∘C below the critical temperature. |
The robust and precise temperature control and uniformity of the freeze drying equipment—a hallmark of Sieno Freeze-drying Technology Research Institute (Jiangsu) Co., Ltd's independently developed intelligent systems—is essential here. Their specialized control systems, developed through strategic collaborations with food science experts, can execute the complex thermal profiles and maintain the deep vacuum required to sustain sublimation efficiently without compromising the integrity of the frozen specialty chemical product.
Secondary Drying Stage (Desorption)
Once the bulk of the ice is removed, the goal shifts to removing the remaining, adsorbed moisture.
- Removing Residual Moisture: The shelf temperature is gradually increased, and the vacuum is maintained or slightly reduced. This provides energy to break the molecular bonds between the water molecules and the dry product matrix.
- Optimizing Temperature and Pressure: This stage is optimized to achieve the desired extremely low moisture content (often <1%), which is critical for long-term stability and shelf life. The process must balance the need for low moisture with the risk of thermal degradation at higher temperatures.
Post-Freeze Drying Steps
The cycle is not complete until the product is safely contained.
- Backfilling with Inert Gas: Before the vacuum is released, the chamber is often refilled with an inert gas (e.g., nitrogen or argon) to prevent oxidative degradation upon opening.
- Stoppering Vials: Vials are sealed while still under vacuum or the inert gas atmosphere, a function often integrated directly into the production freeze dryers.
- Sealing and Packaging: The final packaged product is prepared for storage and distribution.
Optimizing Freeze Drying Parameters
Process optimization is the phase where science meets engineering, ensuring that the specialty chemicals freeze drying equipment operates at peak efficiency while guaranteeing product quality for sensitive specialty chemicals. Optimization requires fine-tuning formulation, freezing, and drying protocols.
Formulation Optimization
The formulation determines the physical behavior of the product during freezing and drying. Optimizing it minimizes stress on the active ingredient and maximizes final product stability and performance.
- Selecting Appropriate Cryoprotectants:
- Goal: To protect the structure of the active ingredient (e.g., a protein therapeutic or enzyme) during the freezing stage and maintain a stable, non-collapsed cake structure during drying.
- Common Entities: Sucrose, trehalose, and mannitol are widely used. Trehalose is often preferred for biologics due to its superior ability to stabilize proteins and maintain cell viability (probiotics) by replacing water molecules.
- Optimizing Solute Concentration and pH:
- The concentration of excipients dictates the critical temperatures, such as the eutectic point or glass transition temperature.
- The pH impacts the charge and stability of proteins and other sensitive molecules, requiring buffer systems that maintain the optimal pH throughout the process.
Freezing Protocol Optimization
The freezing protocol dictates the ice crystal size and distribution, which directly affects the resistance to mass transfer during primary drying.
- Determining Optimal Cooling Rates:
- Impact: Determines the size of ice crystals. Slower cooling yields larger crystals, potentially shortening primary drying time but increasing the risk of cryo-damage. Faster cooling yields smaller crystals, necessary for maintaining the integrity of nanomaterials, but increases drying time.
- Optimization: A cooling rate is selected that balances product stability, desired morphology, and efficient drying time.
- Annealing Cycles:
- Purpose: Heating the product to just below its melting point for a short duration and then re-cooling. This promotes the growth of smaller, unstable crystals into larger, more stable ones, which can reduce primary drying time by $20\%$ to $50\%$ in some cases without increasing product degradation.
Drying Cycle Optimization
Optimizing the drying cycle is about maximizing the heat input (shelf temperature) while ensuring the chamber vacuum maintains the product temperature below its critical collapse temperature.
| Parameter |
Impact of Increase |
Optimization Goal |
| Shelf Temperature |
Increases sublimation rate (faster drying); Increases risk of collapse if too high. |
Maximize heat input without letting the product temperature exceed the critical temperature. |
| Chamber Pressure |
Increases product temperature (slower sublimation); Increases mass transfer efficiency at low pressures. |
Find the highest allowable pressure that maintains a deep vacuum and keeps the ice interface sufficiently cold. |
| Drying Time |
Increases energy consumption; Improves residual moisture removal. |
Shorten primary drying for efficiency; Extend secondary drying to reach ultra-low moisture content for stability. |
- Using Process Analytical Technology (PAT):
- Advanced freeze drying equipment incorporates PAT tools (e.g., Tunable Diode Laser Absorption Spectroscopy - TDLAS, capacitance manometers, and thermocouples) to monitor the process in real-time.
- This allows for continuous monitoring of product temperature and water vapor flow, enabling dynamic adjustment of shelf temperature and pressure to ensure the product is dried as fast as possible without risk of collapse, leading to significant process optimization.
Sieno Freeze-drying Technology Research Institute (Jiangsu) Co., Ltd, recognizing that effective drying of high-value specialty chemicals hinges on this precise thermal and vacuum control, has invested heavily in developing tailored solutions and independently developed intelligent freeze-drying equipment. Sieno’s expertise, consolidated through strategic collaborations with university food science schools, allows them to provide food enterprises—and by extension, the specialty chemical sector—with comprehensive technical support spanning from raw material processing to finished product packaging. This ensures clients can efficiently transition optimized laboratory protocols to large-scale, automated production cycles, empowering them to achieve efficiency gains and quality control upgrades.
Applications of Freeze Drying in Specialty Chemicals
The utility of freeze drying (lyophilization) spans numerous sectors within the specialty chemicals industry, driven by its capacity to stabilize sensitive and high-value materials. This process is essential for materials where maintaining activity, viability, or precise structural integrity is non-negotiable.
Pharmaceuticals
Freeze drying is foundational to the development and manufacturing of modern biopharmaceuticals, ensuring the long-term stability and efficacy of complex drug products.
- Vaccines and Injectables: Many modern vaccines, especially live attenuated or subunit vaccines, require low-temperature processing. Freeze drying preserves the biological activity and structure of these active ingredients, allowing for storage and transport without continuous deep-freeze requirements, significantly extending their shelf life.
- Protein Therapeutics and Antibodies: Large biological molecules, such as monoclonal antibodies and recombinant proteins, are highly susceptible to denaturation from heat and water. Lyophilization removes water gently, replacing it with stabilizing agents (like trehalose or sucrose) to maintain the protein's tertiary structure and therapeutic function upon reconstitution.
Diagnostics
In the diagnostics field, accuracy and reliability are paramount, making freeze drying a critical tool for reagents and calibrators.
- Enzymes, Antibodies, and Calibrators: Diagnostic test kits often rely on highly sensitive biological reagents like enzymes or antibodies to detect specific biomarkers. Freeze drying stabilizes these components, ensuring their activity remains consistent over time. Calibrators and controls are lyophilized to ensure a precise, non-degrading standard for assay validation.
- Benefits: The process ensures the high purity, consistency, and stability needed for reliable and reproducible diagnostic results across different batches and geographies.
Probiotics and Enzymes
Maintaining the biological function and cell viability of living organisms and biocatalysts is a key application area for lyophilization.
- Probiotics: Preserving Viability and Stability of Microorganisms: Live bacterial cultures are extremely sensitive to heat and moisture. Freeze drying is the preferred method for commercial probiotic supplements as it significantly reduces the moisture content to an inert level, placing the microorganisms in a state of suspended animation. This maximizes cell viability over the product's shelf life.
- Enzymes: Maintaining Activity and Stability: Industrial and pharmaceutical enzymes (biocatalysts) lose activity if their structural integrity is compromised. Lyophilization allows enzymes to be stored as a dry powder, ready for immediate use in chemical reactions or assays without the performance degradation associated with liquid storage or conventional drying.
Nanomaterials and Advanced Materials
Freeze drying is increasingly employed in advanced materials science to create products with controlled morphology and structure.
- Creating Stable Dispersions and Controlled Particle Sizes: Techniques like freeze drying prevent the agglomeration or clumping of nanomaterials (e.g., liposomes or carbon nanotubes) that can occur during evaporation. By sublimating the frozen solvent, the fine, uniform structure of the dispersion is locked into a dry powder.
- Controlled Morphology: The structure of the final lyophilized cake can be influenced by the freezing protocol, allowing researchers to control porosity and surface area, which is vital for drug delivery systems and advanced catalytic supports.
The application of freeze drying across these high-stakes industries requires not just standard equipment but intelligent equipment manufacturing tailored for complex thermal and vacuum profiles. Sieno Freeze-drying Technology Research Institute (Jiangsu) Co., Ltd exemplifies this specialization. Sieno’s foundational work, which includes the profound integration of freeze-dried food science with intelligent equipment manufacturing and leveraging world-foremost freeze-drying technology resources, ensures they can provide the robust, highly controlled freeze drying equipment necessary to stabilize and preserve the most delicate entities, from probiotics to complex protein therapeutics.
Best Practices for Freeze Drying Specialty Chemicals
Achieving consistent, high-quality results when freeze drying sensitive specialty chemicals requires strict adherence to best practices in development, equipment management, quality control, and compliance.
Process Development and Scale-Up
Transitioning a successful lab-scale formulation to a commercial product is a complex task demanding rigorous science and engineering.
- Conducting Thorough Feasibility Studies and Process Optimization: Initial studies must accurately determine the critical product temperatures using techniques like freeze-dry microscopy or differential scanning calorimetry (DSC). The resulting data is used to define the safe operating window for shelf temperature and chamber vacuum, ensuring process optimization before committing to large-scale runs.
- Using Scale-Up Models to Predict Performance at Larger Scales: Heat and mass transfer are dramatically different in a small vial on a laboratory freeze dryer versus hundreds of vials in a production freeze dryer. Scale-up models (based on vial heat transfer coefficient $K_v$) are used to accurately predict primary drying time and temperature profiles, allowing the larger equipment to execute the optimized lab cycle effectively.
Equipment Qualification and Validation
To meet cGMP requirements, all freeze drying equipment must be systematically verified and proven to perform according to specifications.
- Ensuring Equipment Meets Performance Specifications and Regulatory Requirements: This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). These steps verify that the vacuum system, refrigeration system, and control system all function within the required tolerance range.
- Validating the Freeze Drying Process to Demonstrate Reproducibility and Consistency: Process Validation proves that a specific lyophilization cycle consistently produces a product that meets all quality attributes (e.g., target moisture content and stability) across multiple batches and over time.
Quality Control and Assurance
Robust quality control procedures are mandatory for high-value specialty chemicals, particularly in pharmaceuticals and diagnostics.
- Implementing Robust Quality Control Procedures: This involves monitoring in-process parameters (e.g., TDLAS data, chamber pressure charts) and post-process product attributes.
- Using Analytical Techniques to Characterize the Freeze-Dried Product:
- Residual Moisture Content: Measured typically by Karl Fischer titration or thermogravimetric analysis (TGA). Crucial for predicting long-term shelf life. Target is often <1%.
- Stability: Testing involves accelerated and real-time stability studies to ensure the product (e.g., vaccines, enzymes) maintains activity over its intended life.
- Reconstitution Time and Appearance: A fast, clear, and complete reconstitution is a key quality attribute, indicating a proper, non-collapsed lyophilized cake structure.
Troubleshooting Common Issues
Anticipating and quickly resolving common issues minimizes batch loss and downtime.
| Common Issue |
Likely Cause |
Best Practice Solution |
| Cake Collapse or Melt-back |
Product temperature exceeded the critical collapse temperature during primary drying. |
Lower the shelf temperature; Increase the chamber vacuum; Use PAT (TDLAS/thermocouples) for better temperature monitoring. |
| Poor Reconstitution |
Non-optimal freezing (too fast/too slow) or excessive primary/secondary drying leading to a dense product structure. |
Optimize cryoprotectants in the formulation; Adjust the cooling rate or implement an annealing cycle. |
| Loss of Activity/Potency |
Thermal or cryo-damage occurred during freezing or secondary drying. |
Use more effective stabilizers (trehalose); Lower the final secondary drying temperature. |
Regulatory Considerations
Compliance with global health authorities is necessary for any specialty chemical intended for human or animal use.
- Understanding and Complying with Relevant Regulations: All activities, from formulation to final packaging, must adhere to guidelines set by authorities like the FDA, EMA, and local regulatory bodies. This encompasses cGMP requirements for manufacturing, material traceability, and process documentation.

 English
English  русский
русский  中文简体
中文简体 
























 +86-180 6875 7376
+86-180 6875 7376  +86- (0) 519-8578 6988
+86- (0) 519-8578 6988  emmy@jsblk.com
emmy@jsblk.com  Zhenglu Town, Tianning District, Changzhou City, Jiangsu Province, China
Zhenglu Town, Tianning District, Changzhou City, Jiangsu Province, China